The Basic Science Award 2017 to a UniSR Research; Margherita, our PhD student, among the authors

The European Blood and Marrow Transplantation (EBMT) is the reference company in Europe that deals with bone marrow transplantation and collects all clinicians, doctors, researchers, European marrow transplanters; each year it assigns the Basic Science Award, a prestigious award for basic research in blood or marrow transplantation. This year the award was assigned to Dr. Attilio Bondanza research group, a former researcher at the Vita-Salute San Raffaele University, a transplant doctor and Principal Investigator of the “Innovative Immunotherapy” Unit. The reason for this recognition was “For the contribution to the advancement of transplantation science and genetic immunotherapy for cancer”. Dr. Bondanza’s group has in fact developed an innovative animal model to study the effects of CAR-T lymphocytes, which allows you to recap exactly what happens during the disease in humans: the use of CAR-T on humans has been developed for years, but this model can be used to predict effectively what so far has been observed only experimentally on humans.
The award-winning study is the subject of PhD thesis by Margherita Norelli, a former Biotechnology student at UniSR and now PhD graduate at our Molecular Medicine PhD programme. In this article we asked Dr. Norelli and Dr. Bondanza to illustrate what their research is about.
What are CAR-T cells?
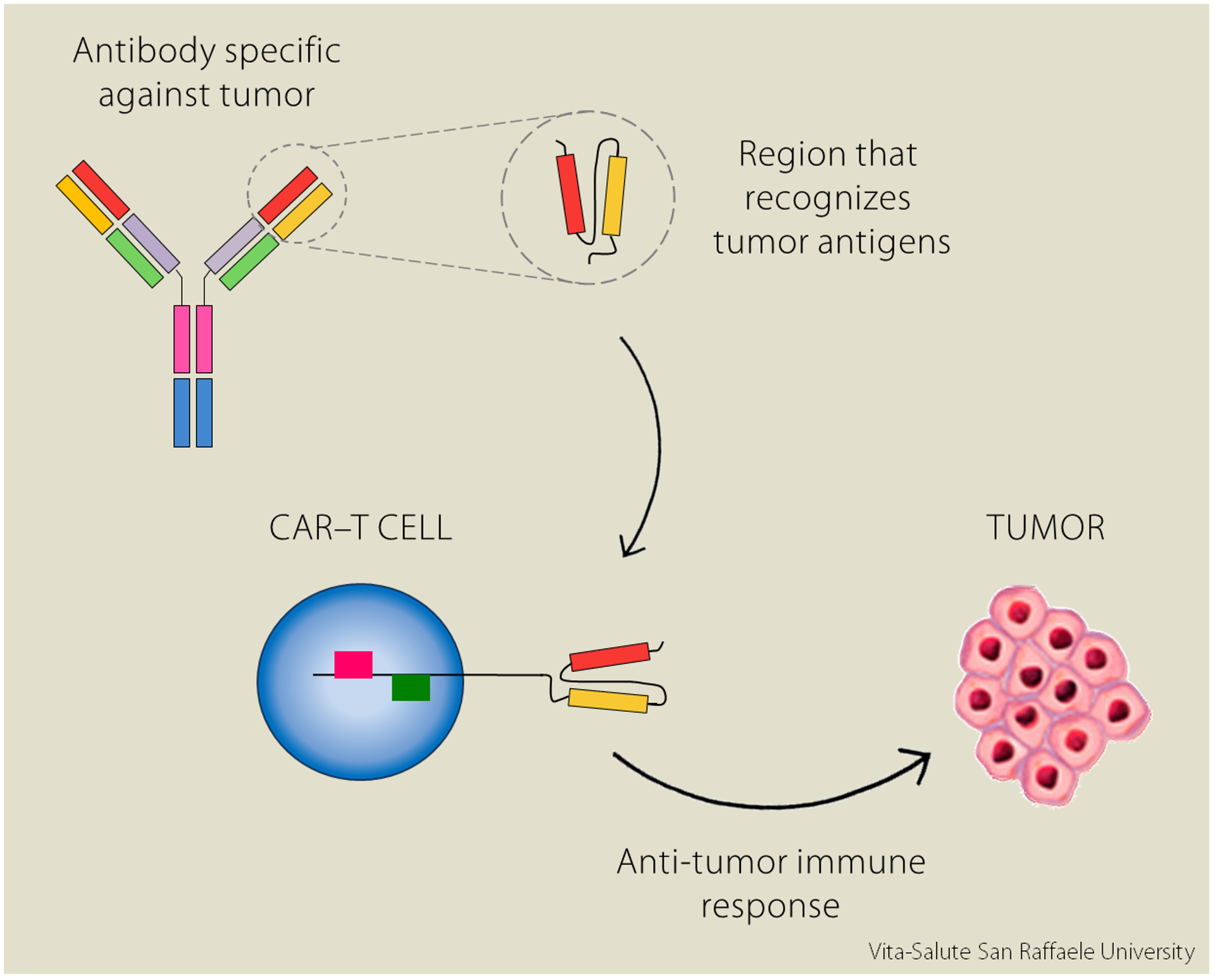
Some cells in our immune system produce some proteins called antibodies. Each antibody is specific in recognizing a particular pathogen, a potentially dangerous substance; similarly, there are also antibodies capable of recognizing tumors.
“In the laboratory” explains Dr. Norelli “it is possible to isolate the variable regions of the antibody [red and orange in the image, editor’s note], the regions that recognize in an extremely specific way the molecules expressed on tumors surface”.
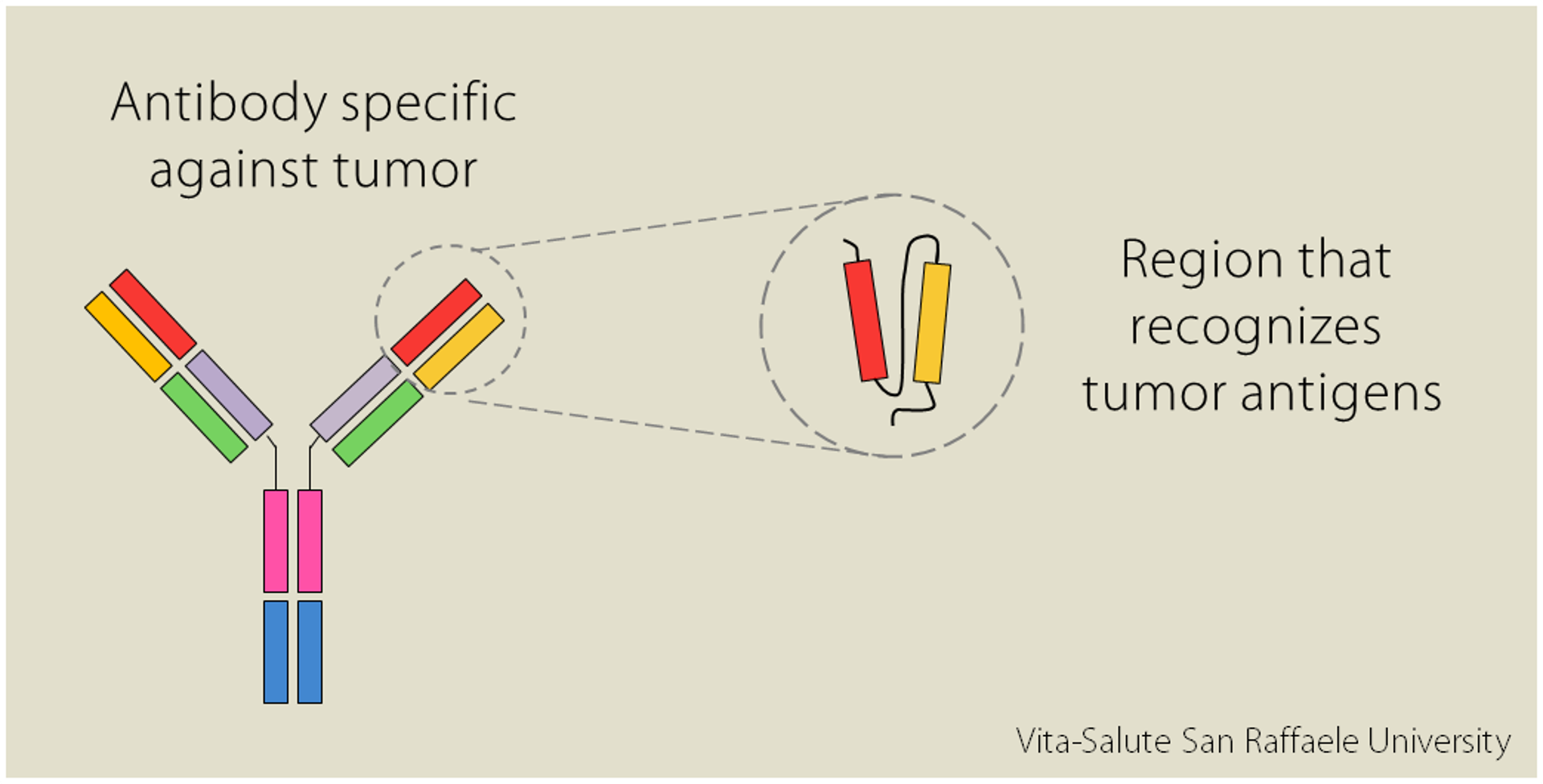
The intuition was to combine the specificity of the antibody to recognize the tumor with the efficacy of T lymphocytes, the cells of our immune system that attack foreign substances: by modifying them, it is possible to “educate” them to recognize harmful organisms and to potentiate their defensive response. “The idea of CAR-T cells was born like this: T lymphocytes modified so as to express receptors created in the lab. The acronym “CAR” stands for Chimeric Antigen Receptors; they are called “chimeric” because they are composed of several elements:
- the variable region originated from the antibody, which recognizes antigens expressed by the tumor
- a spacer, linking region
- the intracellular signaling domains within the T lymphocytes”.
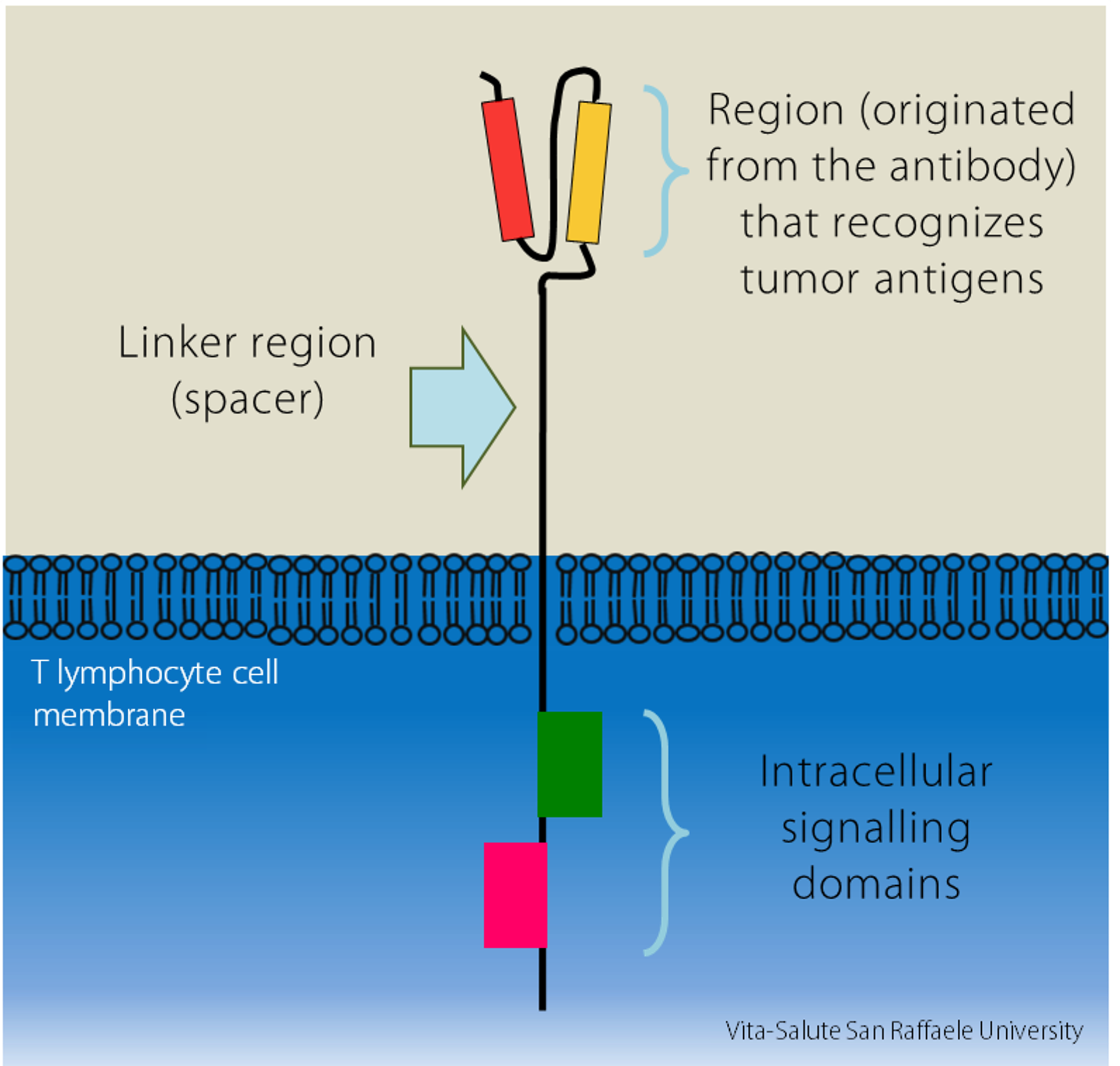
CAR-T cells are therefore genetically engineered cells to express the chimeric receptor on their surface, so they recognize tumors more specifically and efficiently.
“In the study we presented at the Congress, we tested these CAR-T cells on acute myeloid leukemia cells (AML), a blood cancer”.
Similar experiments had already been conducted in the United States, using CAR-T cells against B cell leukemias. “However, these studies saw an unexpected event emerging: there was a severe toxicity in patients, called cytokine-release syndrome (CRS)”. This effect is triggered when T lymphocytes recognize the tumor and release an immense amount of pro-inflammatory cytokines (molecules).
What is new about this research?
Dr. Norelli’s interest was to understand what molecular mechanism caused this violent cytokine storm: to do so, she generated a preclinical animal model that provided the basis for improving human therapy.
“Very often the results of animal studies, once applied to clinic, are ineffective or inadequate; the first thing we did was to create a mouse model with an immune system identical to humans. This model is called “humanized” because the mouse acts as a guest, almost as a “container” of human cells”.
In the study, mice were transplanted with human hematopoietic stem cells which will give rise to all human immune system, simultaneously injected with human tumor cells and treated with human CAR-T cells.
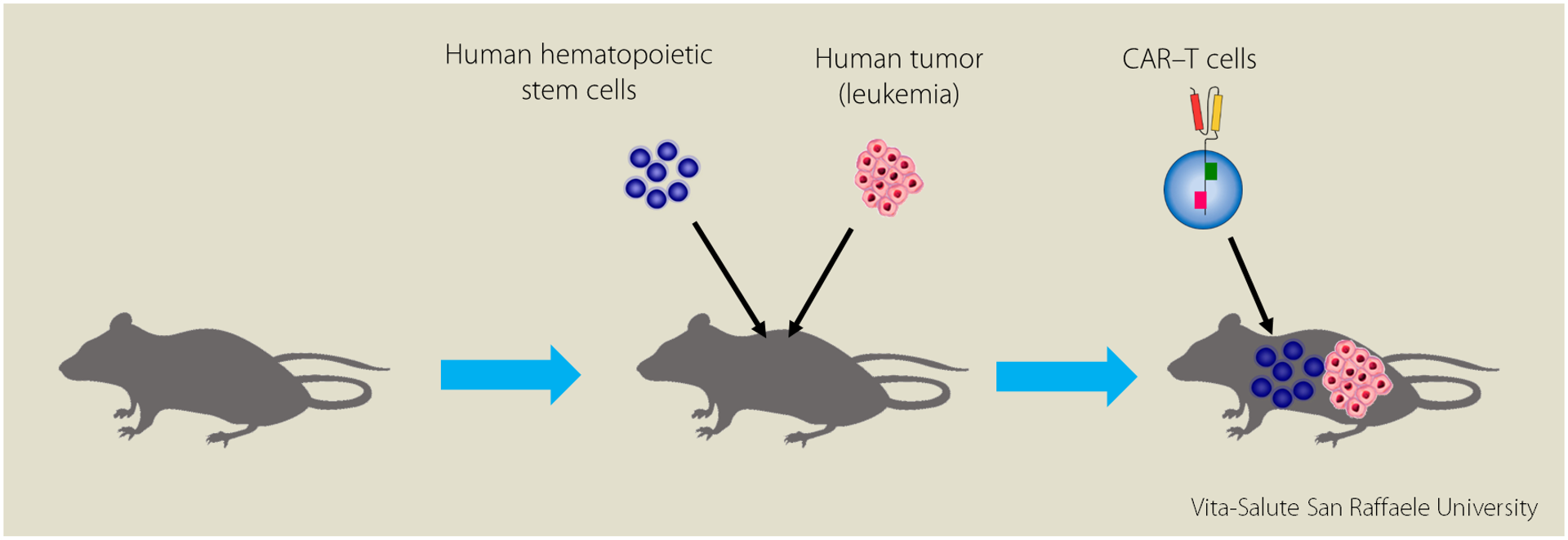
“Despite the negative effect” Dr. Norelli continues “this cytokine stomach would seem useful, if not in some cases fundamental, to give a second activation to T cells and thus contribute to eradication of the tumor”.
How then to cause the anticancer effect without generating fatal toxicity for patients?
“We have decided to test two drugs (called Tocilizumab and Anakinra), already used in the clinic to block the action of some inflammatory cytokines: we used them to assess whether or not they can fight toxicity. A group of mice were given these drugs simultaneously with the infusion of CAR-T cells; a second group of mice acted as a control, and did not receive the drugs. Weekly we followed them to evaluate the possible development of toxicity and tumor status”.
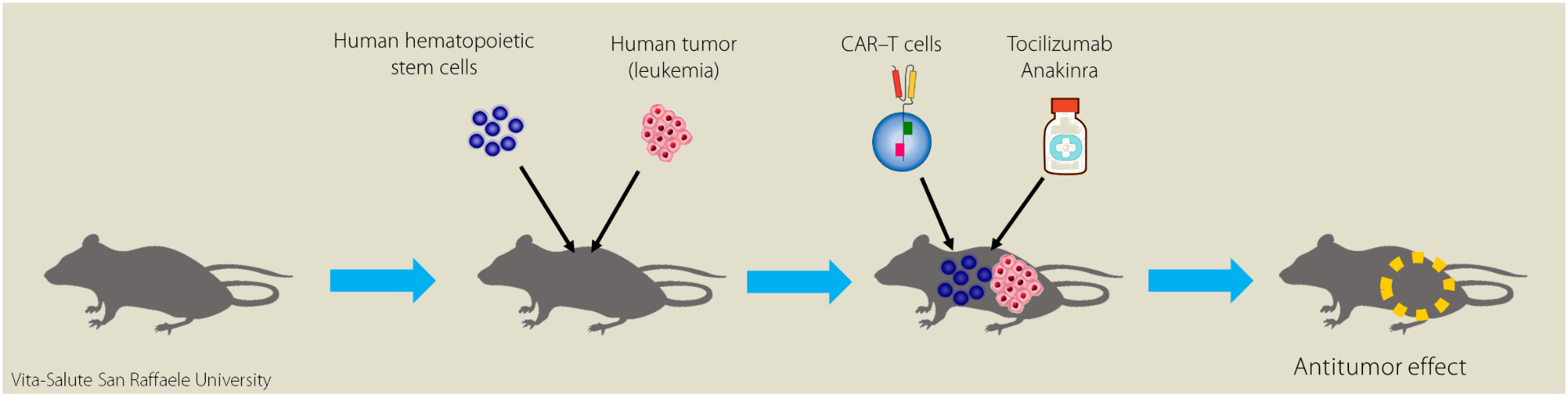
“At the end of the study” Dr. Bondanza adds, “we observed that mice that received these two drugs in addition to the CAR-T cells give the positive result of cancer eradication without causing the adverse effect of toxicity. This innovative approach is an important strategy for enhancing anti-tumor activity without affecting the activity of CAR-T cells”.
“This kind of study cannot be performed in humans, so the use of the animal (which is treated with great respect and in accordance with the Regulation of the Institutional Commission for the Care and Use of Animals) is unfortunately necessary” concludes Dr. Barbara Camisa, a fundamental contribution to in vivo studies. “Only in this way we could realistically imitate the molecular mechanisms occurring in humans, with the result that, by using these therapies, it is possible to mitigate and abolish the very severe toxicity development and to maintain the anticancer effect for a long time” .
Results that make us really hope for the best.
You might be interested in

Multiple sclerosis, breakthrough in research: researchers have identified a molecule that promotes repair of the nervous system
The microbiome as an ally against myeloma

Intrecci: a UniSR project for more inclusive and accessible cancer diagnosis