UniSR research will deepen the study of plasma cells in systemic amyloidosis

Plasma cells are cells of the immune system, responsible for the production of antibodies against pathogens; systemic amyloidosis is a rare disease that affects such cells. A UniSR study will explore the cellular factors behind the remodeling of the secretory apparatus of plasma cells, with the long-term goal of identifying a more targeted and effective therapy for the treatment of the disease.
The project is coordinated by Enrico Milan, a young UniSR researcher, in partnership with Dr. Mario Nuvolone of the IRCCS Policlinico San Matteo di Pavia Foundation. The proposal was the winner of a Cariplo “Biomedical Research Conducted by Young Researchers” funding, which offers researchers under 40 the opportunity to develop and enhance independent careers, conducting research projects under their own responsibility. “This Cariplo funding is essential to allow me to develop my studies and help me in an important transition moment in my career towards independence. For the first time, it guarantees me greater stability and the possibility of planning more lasting, articulated and ambitious projects” declares Enrico. “In addition, this financing has allowed the creation of a collaboration between two centers of excellence of Lombardy research, joining our team of plasma cell biology with the group of Policlinico San Matteo, world excellence as regards the study and care of systemic amyloidosis”.

Systemic amyloidosis from immunoglobulin light chains
Before introducing Enrico’s research, let’s do a quick review of some elements of the immune system. B lymphocytes are cells that play a primary role in the defense against pathogens: when they encounter an antigen, i.e. a foreign and potentially dangerous substance, they activate and undergo a complex process of cell differentiation, giving rise to plasma cells. These cells are specialized in the production of antibodies against pathogens. Most of them are short-lived; some plasma cells, however, persist even until old age in specialized niches of the bone marrow. Thanks to the latter, researchers are able to readily recognize the same pathogen when it recurs: it is one of the mechanisms of immunological memory.
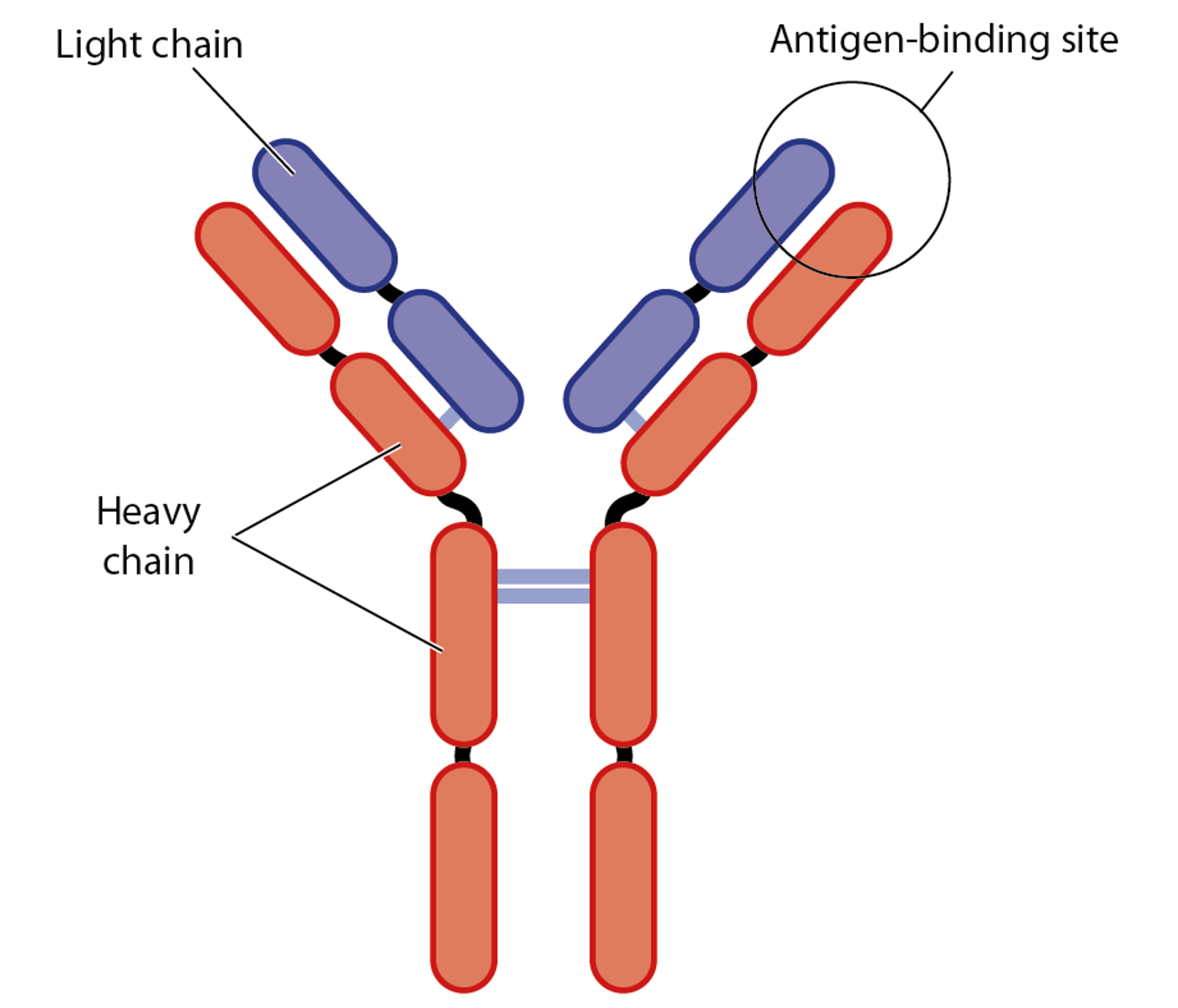
The antibodies that the plasma cells produce are Y-shaped molecules, and are composed of two heavy chains (in red in the drawing) and two light chains (indicated in purple).
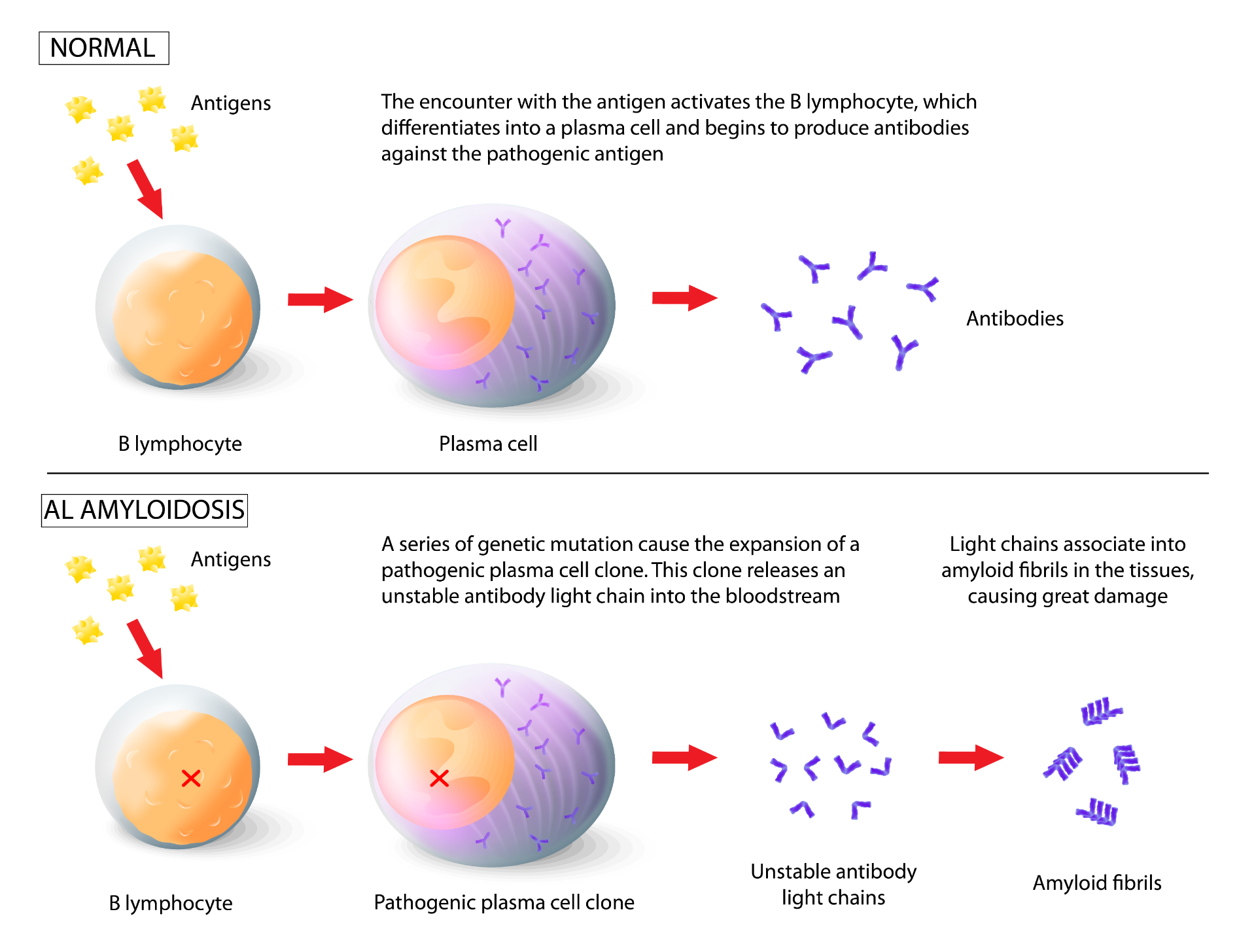
Enrico’s study concerns systemic amyloidosis from light chain immunoglobulins (or “AL amyloidosis”), a rare disease that affects plasma cells. He says: “This disease is caused by the expansion of a plasma cell clone [i.e. a group of identical cells that derive from a common ancestor, Editor’s note], which releases an unstable antibody light chain into the bloodstream. This unstable chain aggregates in the form of extracellular deposits called amyloid fibrils, which accumulate in the tissues and damage them. If left untreated, AL amyloidosis is inevitably fatal mainly due to heart damage due to the accumulation of these fibrils. Other organs, such as the kidneys, liver, gastrointestinal tract and nerves, may also be affected during AL amyloidosis”.
“The main therapy for this pathology is based on chemotherapy, possibly associated with the autotransplantation of blood stem cells, and it aims at eradicating the pathogenic clone. However, in many cases the plasma cell clone persists despite therapy, or it returns after an initial phase of remission of the disease, due to phenomena of resistance to the drugs used”. Future prospects therefore aim to study and learn more about the cellular characteristics underlying this pathology, in order to discover new weaknesses and identify a more targeted and effective therapy.
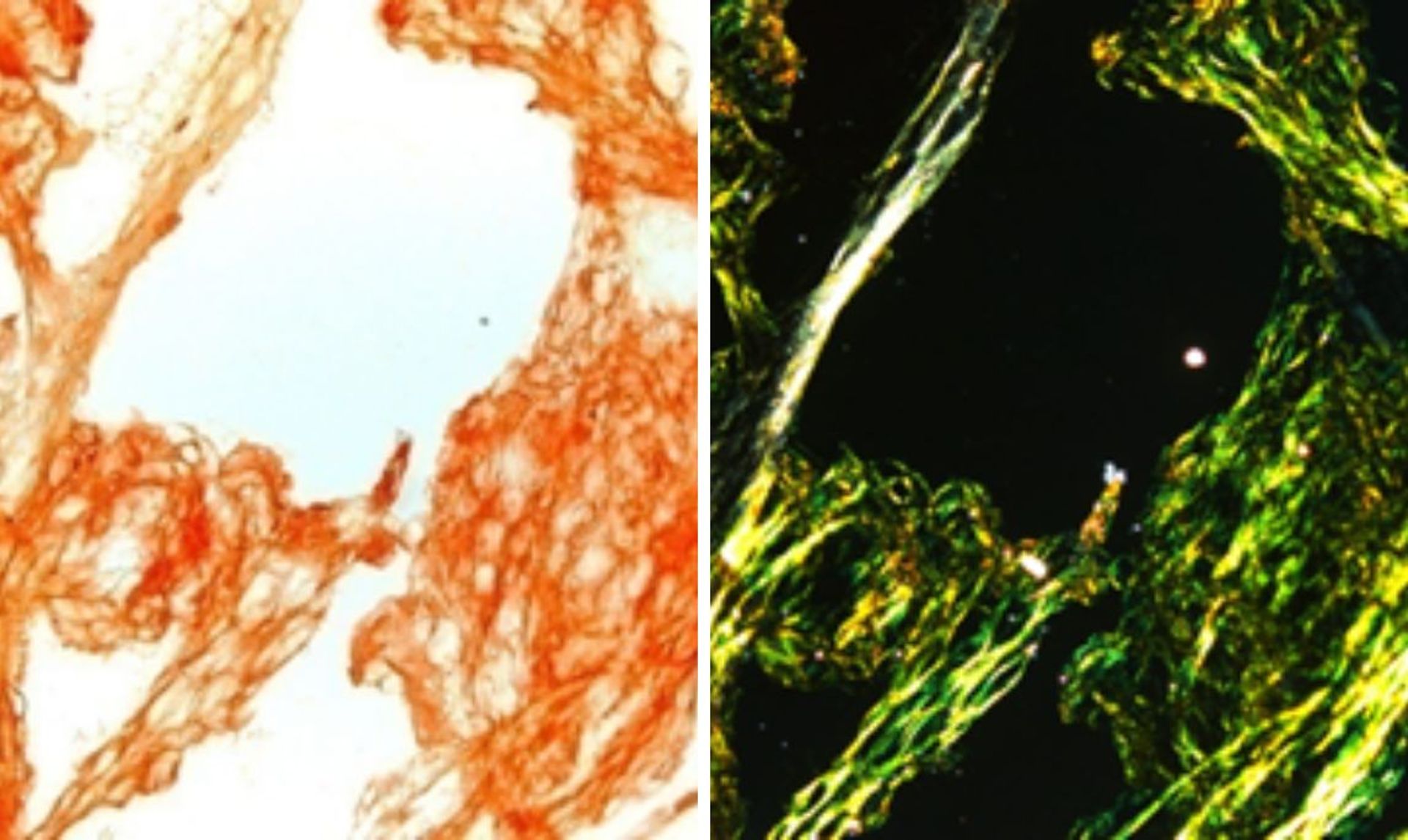
Amyloid deposits stain in red upon staining with Congo red (left), while they show a characteristic apple green birefringence under polarized light (right). Courtesy of IRCCS Policlinico San Matteo di Pavia Foundation.
The fascinating “antibody factory”
“Plasma cells originate from B lymphocytes thanks to a complex cell remodeling that allows them to enhance the secretory system, so as to allow a massive and immediate release of antibodies against the pathogen. I have always been fascinated by the study of these cells with such a unique biology, such as to make them a real “antibody factory” - says Enrico, postdoc in the “Age-related Diseases” Unit, directed by Dr. Simone Cenci (he himself winner of a Cariplo Funding), where for years they have been studying the biology of plasma cells and their neoplastic form: Multiple Myeloma.
Plasma cells need a perfect balance among energy resources, quality control systems for protein synthesis and folding and the ways to dispose of damaged antibodies. “In this context, we have always been concerned with exploring how plasma cells maintain the balance of protein homeostasis in order to discover new possible therapeutic targets that could block the assembly line of this biological “factory”. Among the possible targets we have studied there are the proteasome and autophagy, the two main systems of degradation of cellular waste, essential for the survival of plasma cells. In fact, proteasome inhibitors have radically changed the clinical treatment of myeloma and AL amyloidosis. Unfortunately, many times resistance to these drugs arises and the search for new therapeutic targets is therefore still necessary. With our studies, we try to understand how to increase the effectiveness of existing drugs and identify new potential therapeutic targets. Furthermore, discovering in detail all the mechanisms underlying how antibodies are produced is extremely relevant in many aspects, beyond plasma cell diseases – just think of the role of antibodies in infectious, autoimmune diseases and their possible use as new biotechnological drugs”.
Enrico's project
The project that Enrico will conduct in collaboration with Dr. Nuvolone will deal with exploring the molecular mechanisms that allow the plasma cells to remodel their secretory system and survive the very high levels of stress to which they are subjected. “In particular, we will study the cellular factors responsible for the expansion of the secretory pathway during differentiation from B lymphocytes and the mechanisms by which this activity is coordinated with degradative processes. In this way, we hope to find new systems that allow us to break this delicate but fundamental balance, thus eliminating the clone that releases unstable antibody light chains, or at least reducing their ability to secrete toxic antibody. In addition, we will extend our analysis to rare samples of AL amyloidosis patients by focusing on possible mutations acquired by the plasma cell clone and how these mutations can affect drug sensitivity”.

Dr. Nuvolone's team
Tags:
You might be interested in

UniSR ranked 1st in Italy for Research and Teaching
/resolutions/res-c660x528/Veschetti_Cariplo_P.-Aeruginosa_UniSR-(1).jpg)
Uncovering the hidden role of bacterial microRNAs in chronic respiratory diseases
A New Approach to Enhance Immunotherapy in Multiple Myeloma

