Thanks to the collaboration with Intellia Therapeutics, the first clinical trial of the investigational cell therapy utilizing a WT1-targeting T cell receptor developed in San Raffaele’s labs has received regulatory approval in the United States and the United Kingdom and began enrolling patients with acute myeloid leukemia (AML) resistant to current treatments
Milano, 9 February 2022 – A new type of engineered lymphocytes designed to recognize acute myeloid leukemia cells with high precision and to remain in circulation longer, ready to reactivate in case of relapse.
These are the features of the innovative experimental cell therapy utilizing a Wilms Tumor 1 (WT1)-targeting TCR born in the laboratory of Chiara Bonini, deputy director of the Research Division in Immunology, Transplantation and Infectious Diseases of IRCCS Ospedale San Raffaele in Milan and full professor of hematology at the Vita-Salute San Raffaele University.
The result was obtained thanks to the collaboration with the U.S. company Intellia Therapeutics, leader in the field of CRISPR/Cas9 technology. Ospedale San Raffaele and Intellia have a strategic partnership to accelerate the development of a WT1-targeting TCR-based T cell therapy.
Based on the safety and efficacy results obtained in preclinical models of the disease, published today in the prestigious journal Science Translational Medicine and expanded upon by Intellia, the company has already obtained the green light from regulatory bodies in the U.S. and U.K. to start the first clinical trial in patients with acute myeloid leukemia.
A new technology to boost the immune system
The technology, used by San Raffaele and Intellia researchers to engineer lymphocytes, consists of molecular scissors (CRISPR/Cas9) to replace the receptors naturally present on their surface – called T Cell Receptors (TCR) – with other TCRs, previously isolated from the blood of healthy subjects, for their ability to recognize a specific tumor protein, in this case WT1. The result is an army of highly tumor-specific T lymphocytes.
“TCRs are very powerful and versatile tools. With these receptors, the engineered lymphocytes are able to identify a tumor cell not only on the basis of the surface proteins it possesses (as in the case of CAR-T therapies), but also on the basis of proteins or other types of molecules that are used inside the tumor cell."
- said Eliana Ruggiero, a researcher working in Chiara Bonini's laboratory and first author of the study.
Adds Ruggiero:
“This has a lot of advantages. First of all, it expands the number of neoplasms we can treat, because it expands the number of tumor molecules and proteins that we can target. It is also easier to find internal molecules essential for the survival of the tumor, which means molecules that the tumor cannot replace or eliminate in order to escape the therapy. This is how we chose our target, WT1, a fundamental protein for acute myeloid leukemia cells, so much so that it is already used today in the clinic as an indicator of the severity of the disease and to assess the risk of relapse."
In addition to all of this, TCRs, being those physiologically present on lymphocytes, are also able to stimulate immune memory mechanisms. When activated by their antigen, these receptors promote the survival of T cells, which remain in circulation, ready to proliferate and get back to action in case the threat reappears, such as during a relapse, a frequent occurrence in acute myeloid leukemia.
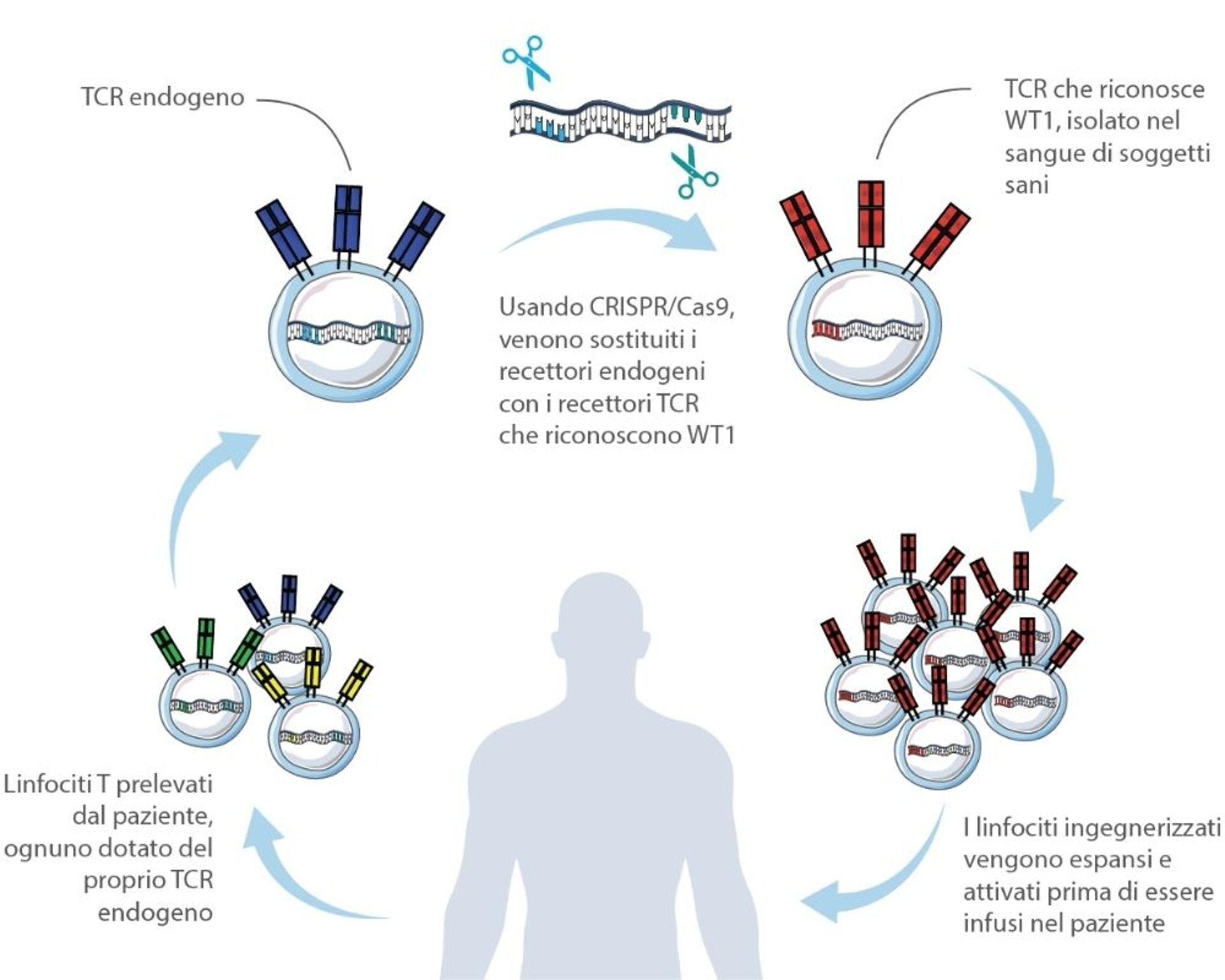
How to solve the compatibility problem: Building a receptor library
Compared to the CAR-T approach, TCR-engineered lymphocytes have particular features that make the technology more complex to handle and the development of potential therapeutic options accessible to all patients longer.
“There are already challenges to overcome in the development of cellular therapies with engineered T lymphocytes, no matter what technology is used. First of all, we need to identify the target protein, in our case WT1, then we need to find the receptors able to recognize it. To this end, we literally go hunting for the lymphocytes that possess them in blood samples donated by healthy subjects."
- said Prof. Chiara Bonini.
Adds Fabio Ciceri, professor of Hematology at the Vita-Salute San Raffaele University and Head of the Hematology Unit of IRCCS Ospedale San Raffaele:
“In the case of TCR technology –every cell therapy endowed with a specific TCR – can be administered only in a specific group of patients, those with the same histocompatibility as the healthy person from whom the receptor was isolated. This is a situation similar to that of bone marrow transplantation, in which donor and recipient must be compatible. However, unlike in transplantation, in this case compatibility on a single molecule is sufficient."
In the case of the WT1-targeting TCR developed at San Raffaele and described in today’s publication, a TCR capable of recognizing WT1 was chosen among 19 candidates, because of its high avidity and its derivation from a donor with a histocompatibility molecule – called HLA-A*02:01 – which is one of the most common in western countries. But this, nonetheless, means that the experimental therapy can only be tested and then administered in people with HLA-A*02:01.
For other patients, it will be necessary to isolate other TCRs still able to recognize WT1 but compatible with their immune system, an important but temporary limitation. The group led by Bonini has already identified some of these receptors, which are currently being validated.
“The fact that this first receptor will be tested in the clinic makes us proud and excited, but it reminds us that it is only the first step. The ultimate goal is to build a proper TCR library, capable not only of functioning in patients with different classes of histocompatibility but also of recognizing different types of proteins associated with both solid and hematological tumors."
- concludes Bonini.
An ambitious goal, that will be pursued in the coming years also thanks to the support of Fondazione AIRC per la Ricerca sul Cancro.
Tags:
You might be interested in

Multiple sclerosis, breakthrough in research: researchers have identified a molecule that promotes repair of the nervous system
The microbiome as an ally against myeloma

Intrecci: a UniSR project for more inclusive and accessible cancer diagnosis